Supply Chain Intelligence for Pharma
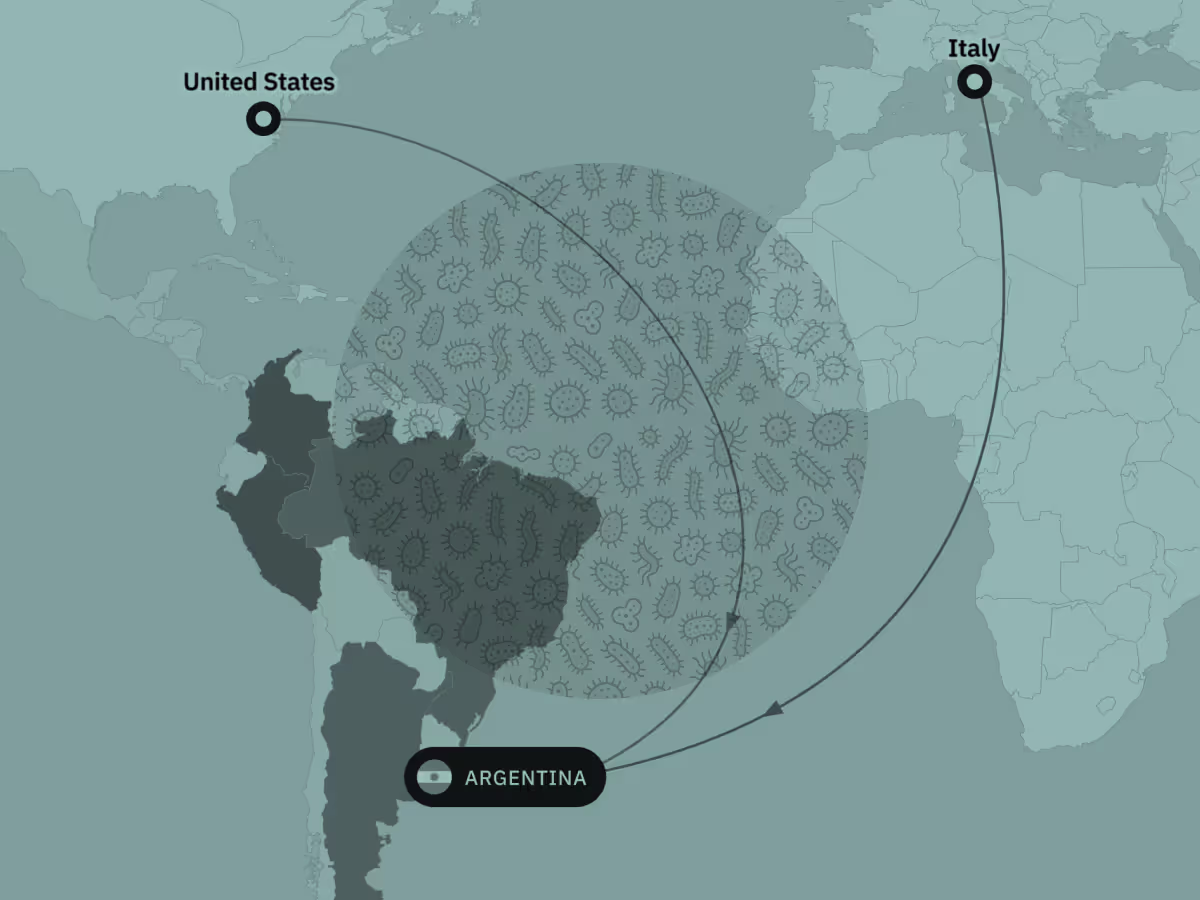
Comparing disease burden data with trade intelligence reveals products moving through informal channels and unmet demand for legitimate brands, offering valuable insights for new commercial opportunities.

Identifying key rare conditions and estimating patient numbers helps define yearly treatment needs, which can be compared with import data to reveal supply gaps. For pharmaceutical companies, strengthening legitimate distribution is both risk management and a strategy for protecting patients and driving growth.

Schedule a free consultation to explore how disease burden and trade data can reveal unmet demand, uncover informal supply channels, and highlight opportunities for growth across the pharmaceutical value chain.
Indicate your product of interest in this form.
Disclaimer: This free consultation offer is available to qualified industry professionals using a valid corporate email address.